CAFO Ammonia: A Major Source of PM2.5 Air Pollution
Nitrogen in manure from confined animal feeding operations (CAFOs) is one of the major sources of water pollution. The major form that nitrogen takes when it is in water is as the ammonium ion (NH4+).
In drain or window cleaner, liquid ammonia exists as an ammonium ion, too. However, when in contact with the air, ammonia gas (NH3) forms. You know it by its choking fumes. Ammonia in gaseous form is a source of air pollution.
You may not know that ammonia generated from manure is also a leading source of particulate matter air pollution, commonly called PM2.5 air pollution. Solid particles 2.5 microns or smaller in diameter, PM2.5 pollution is a silent killer responsible for over four million deaths annually.
In this article, we examine the fate of CAFO ammonia. We focus on how ammonia contributes to PM2.5 pollution in the air we breathe.
Where does ammonia come from?
Ammonia is formed in nature by microorganisms during their physical and chemical breakdown of organic matter (dead plants and animals). The amount of ammonia naturally present, usually in rainwater, from these processes is 1-5 parts per billion (ppb).
Ammonia is also a major industrial chemical synthesized from fossil gas. It is used to make numerous products including chemical fertilizers. As a fertilizer, ammonia or similar substances such as urea, are sprayed on farm fields or injected into soil.
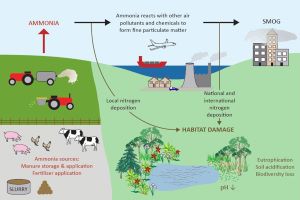
Because of the great inefficiency – up to 80% – in the grain:meat conversion process as we detailed in a previous article, animals excrete waste products high in nitrogen. As microbes decompose the feces and urine, ammonia is released. In CAFOs, ammonia emanates from animal housing, manure lagoons, or the manure sprayed on fields, becoming an air contaminant.
In this article, we use the word manure as the United States Environmental Protection Agency (USEPA) does: “…the combination of manure (i.e., the mixture of feces and urine), water directly or indirectly used in animal production activities…, and other wastes (e.g., hair, bedding, soil, feed) that are mixed with manure.”
Here is an infographic taken from the UK government website showing how ammonia cycles through the environment:
How much ammonia comes from agriculture in the United States?
According to the 2020 National Emissions Inventory (NEI) Report (under the 2020 NEI Updates tab at that link, slide #10), the total amount of ammonia generated from agricultural fertilizer increased 60% in 2020 over 2017 levels (1.8 million tons vs. 1.1 million tons).
This fertilizer is applied to all conventionally grown crops, the large majority of which is animal feed.
How much ammonia comes from livestock in the United States?
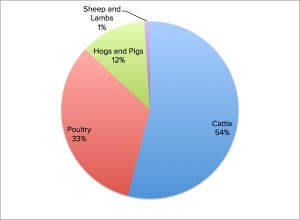
The Ohio State University Extension Service compiled USEPA statistics on ammonia release by various animals from 2004 in a pie chart depiction:
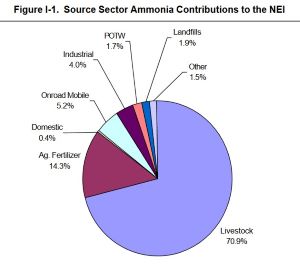
Here is a general overview of 2004 data arranged by USEPA showing a 71% contribution by livestock to national ammonia emissions. Note that an additional 14% is due to agricultural fertilizers, a comparatively smaller portion of which is used for crops consumed by humans. So, roughly 85% of all ammonia emissions in the U.S. in 2004 was derived from agriculture.
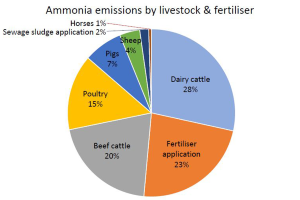
We located a 2004 USEPA document that projected livestock ammonia emissions through 2030. Here is a screenshot of that table:
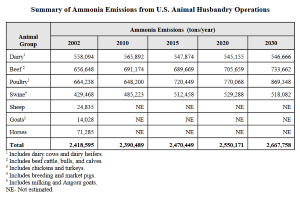 We notice from this projections table that USEPA predicts ammonia emissions between 2020 to 2030 will increase for beef and poultry, stay roughly the same for dairy, and decrease for pigs.
We notice from this projections table that USEPA predicts ammonia emissions between 2020 to 2030 will increase for beef and poultry, stay roughly the same for dairy, and decrease for pigs.
How much does livestock contribute to ammonia emissions in other countries?
In 2016, animal agriculture was responsible for 88% of ammonia emissions in the United Kingdom.
You can see China information at: https://acp.copernicus.org/preprints/acp-2021-439/acp-2021-439.pdf
In China, ammonia emissions came from the sources depicted on the pie chart for 2016. Their breakdown is much more detailed. Ammonia emissions from all fertilizer-related, agricultural, and livestock sources is roughly 91%.
How does ammonia form PM2.5 particle pollution?
Fine particle pollution PM2.5 forms by chemical reactions between gaseous ammonia and sulfuric or nitric acids. These two acids are derived from sulfur oxides and nitrogen oxides (common air contaminants noted as SOx and NOx, mostly from gas- or diesel-powered vehicle exhaust or power plants). The reactions result in ammonium sulfate, ammonium bisulfate, and ammonium nitrate. These compounds exist in the solid state as extremely small yellow or white crystalline particle pollution in air.
A 2011 study published in the Journal of Dairy Science found that ammonia from CAFOs is responsible for, on average, 5-11% of all PM2.5 pollution. The percentage varies widely with higher amounts in the vicinity of CAFOs or downwind from them. It’s also higher in cooler weather. In some areas at certain times of the year, it could be up to 20% of total PM2.5.
Does ammonia cause nitrous oxide to form?
Ammonia is used to manufacture nitric acid, a primary ingredient in fertilizers. That chemical transformation releases nitrous oxide (N2O) emissions.
Nitrous oxide – also used by dentists as laughing gas – is a potent greenhouse gas, contributing to the climate emergency. Most nitrous oxide emissions (74%) come from agricultural soil management which includes:
- Fertilizer application
- Cropping practices
- Manure management
- Burning of agricultural residues
A pie chart on N2O emissions from Inside Climate News based on 2017 data from USEPA can be seen at https://insideclimatenews.org/news/11092019/nitrous-oxide-climate-pollutant-explainer-greenhouse-gas-agriculture-livestock/
What are the health effects of PM2.5 air pollution?
Fine particle pollution known as PM2.5 can reach alveoli deep in the lungs. It may settle there or enter the bloodstream. The pollution particles can cross the blood-brain barrier and damage the central nervous system. Some of the human health effects associated with PM2.5 air pollution include:
- Cardiovascular diseases
- Respiratory conditions
- Lung cancer
- Stroke
- Asthma
- Diabetes
- Premature death
- Low birth weight
What can I do to reduce PM2.5 air pollution caused by agriculture?
Experts say that the more cost-effective way of mitigating agricultural PM2.5 pollution is to reduce ammonia emissions compared to nitrous oxide (N2O) emissions. Doing so will lower the number of premature deaths attributed to PM2.5 and ease environmental harms. It will also go farther in achieving the goal of the Colombo Declaration: halving global nitrogen release by 2030.
So to reduce your personal contribution to agriculturally-produced ammonia, the most important step you can take is choosing a diet that doesn’t include any animal products. However, since all conventionally grown foods rely on some form of fertilizer, the use of which causes ammonia emissions, it will be impossible to eliminate your personal contribution to ammonia pollution completely.
Since the production of chemical fertilizers contributes to greenhouse gas (nitrous oxide) emissions, choosing food grown with natural instead of synthetic fertilizers is preferable. These are foods certified USDA Organic. Note that some natural fertilizers, such as fish meal, are not vegan.
If you grow your own food, or rely on a farmer practicing veganic agriculture, you can best ensure your fertilizer is vegan, optimally applied, and well-timed so as to minimize ammonia volatilization. Then there would be little to no ammonia emissions that could lead to the formation of PM2.5 particle pollution.
To support The Vegetarian Resource Group research, donate at www.vrg.org/donate
Or join at https://www.vrg.org/member/2013sv.php